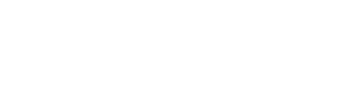Disease and Mycotoxin Considerations for Corn Silage Harvest In Wisconsin
Damon Smith, Extension Field Crops Pathologist, Department of Plant Pathology, University of Wisconsin-Madison
We are quickly approaching that time of year where we will see silage choppers working the 2024 corn crop. This means it is time to understand overall crop health and how diseases might be affecting the crop so that you can make the best silage product you can.
Overall, the corn crop in Wisconsin looks good. Yes, I can find some sort of disease in any field I visit, but I would say for the most part, most fields that were planted relatively on time, and didn’t experience early-season flooding, have a decent crop. With that said, we need to monitor the crop closely as we approach harvest as foliar disease issues can sneak up quickly as cool, wetter weather moves in near the early fall harvest.
Foliar diseases such as southern rust, tar spot, gray leaf spot (GLS), and northern corn leaf blight (NCLB) can be problematic for silage production. Not only do these diseases lead to a reduction in overall silage quality, they can force the plant to scavenge carbohydrates in the stalk which can result in standability issues and lodging. These diseases can also influence whole plant moisture making harvesting at optimal moisture difficult. If silage is harvested at sub-optimal moisture, then packing the bunker properly can be a challenge which can lead to slow fermentation and continued growth of aerobic organisms like fungi. This can indirectly lead to an increase in mycotoxins and “mold” issues from these aerobic fungi.
Tar spot and silage harvest
Tar spot of corn has been an issue on silage corn in Wisconsin since 2016. In fact, the first finding of tar spot was on corn for silage that year. Since 2016 the largest and most consistent impacts happen on corn for silage vs. corn for grain. Yes, tar spot can result in significant grain losses, but tar spot can also affect the overall plant in other ways such as loss in dry-matter yield (Fig. 1) and whole plant moisture contents well below optimal, making bunker-packing a significant issue. In recent work in Wisconsin, we see significant dry-matter yield reductions when tar spot severity on the ear leaf reaches over 10% at the time of chopping. This can result in as much as 15% reduction in yield, with these impacts dramatically increasing at severity levels of 20% or more on the ear leaf (Fig. 1). If tar spot (or any foliar disease) is moving quickly in your silage crop, you might consider chopping a bit earlier to reduce dry-matter yield losses and to try to optimize whole plant moisture, prioritizing good bunker management. If moisture at chopping is not prioritized, then subsequent storage issues such as mold and mycotoxin concerns can arise indirectly, due to poor bunker management. This brings us to Gibberella ear and stalk rot in silage corn.
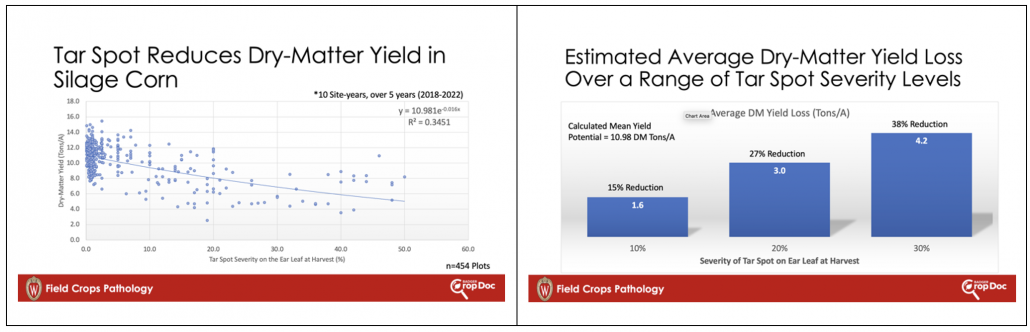
Figure 1. Impact of tar spot severity on the ear leaf at harvest time compared to dry-matter yield in whole-plant chopped silage corn.
Gibberella and silage harvest
Gibberella ear rot is caused by Fusarium graminearum (a.k.a. Gibberella zea). The same pathogen can cause Gibberella crown and stalk rot in corn. We have observed both diseases on silage corn in Wisconsin in recent years. Our environment here in the Great Lakes region makes a perfect place for this pathogen to cause these diseases. In addition to the damage that the pathogen can cause, the fungus can also produce various mycotoxins, most importantly deoxynivalenol (DON or Vomitoxin). Our laboratory has conducted quite a bit of research recently trying to understand where in the plant DON accumulates. In detached plant part experiments, we have noted that DON can accumulate in both the stalk and ear portions of the plant, AND that these two phases of accumulation are not linked to each other (Chibuogwu et al., 2024). The fungus can infect these parts separately at different times during the season and the subsequent accumulation of DON can happen differentially in the stalks vs. the ears. This is partially why you can go out to the field and scout for ear rot and not see a lot of infection (moldy ears), but still have high DON levels at chopping time. Some of that DON is likely accumulating in the stalks.
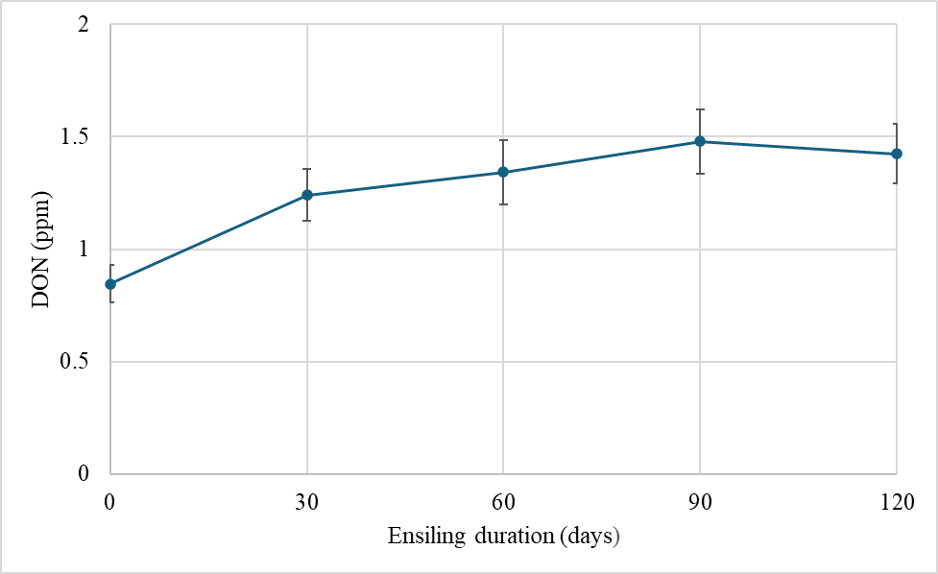
We have also been following the fate of DON in silage harvested and chopped from a brown midrib (BMR) hybrid and a dual-purpose hybrid that were grown in the field and treated with fungicides at white silk (R1). We chopped the plants in each plot and then used mini-silos (polyethylene bags vacuum-sealed using a commercial grade vacuum packer) to conduct a time-course experiment following DON levels in the mini-silos (Chibuogwu et al. 2025). In all cases we saw DON levels generally increasing in the first 30 days after chopping (Fig. 2). They then leveled off and became stable at 60, 90, and 120 days after chopping. Some of this increase could be due to oxygen still in the system during the first 30 days after chopping. DON-producing fungi are aerobic and continue to consume some of the minute levels of oxygen still in the system, thereby still producing DON. However, this likely only explains some of the DON levels we detected.
There are also “masked” or conjugated forms of DON that are detectable in routine DON analyses. One such conjugate is DON-3-glucoside (D3G). D3G can be produced by fungi or during a plant’s attempt to protect itself from the toxicity of DON. Either way, D3G is not detectable in routine test and must be tested for specifically. We investigated our samples further during the first 30 days of ensiling and found that the level of D3G at harvest, explained a significant level of the DON recorded in samples after 30 days of ensiling. This is to say that D3G present at harvest, is likely metabolized in the first 30 days of ensiling releasing DON and resulting in higher DON levels 30-days later (Fig 3). Again, this relationship only partially explains why DON increases in silage during the first 30 days of storage. The full explanation is likely due to both metabolization of D3G and continued fungal respiration leading to an increase in DON at feed out compared to when it was packed in the bunker.
Figure 3. Relationship of DON-3-Glucoside (log) at harvest and DON concentration after 30 days of ensiling.
The Take Home
So, what are we to do with all of this information? Well, knowledge is power. You need to balance foliar disease management with Gibberella/DON management when making high-quality silage in Wisconsin. Diseases like tar spot are the new normal. As you prepare to harvest, it is a good idea to get out in the field and see how bad the foliar disease is and how much ear rot you are seeing. You will want to prioritize harvest on fields showing more disease. In fields where there are high levels of tar spot, southern rust, or NCLB, monitor moisture carefully and try to chop to optimize moisture. Concentrate on good bunker hygiene and spend time packing the material as best you can, focusing on getting as much oxygen out of the system as you can. If a corn crop becomes too dry to make good silage, you might consider harvesting it for high-moisture grain to try to circumvent bigger issues that could arise at feed out, by making less than ideal silage. Finally, it is important to test for DON frequently and understand what you are dealing with. You want to start with the lowest levels of DON coming from the field that you can. DON will likely increase in the bunker no matter how well you pack it. Thus, starting with the lowest levels at harvest will help keep final levels of DON below critical thresholds. Moving forward, mycotoxin testing in corn should include not only DON but also for conjugates of DON that can be metabolized back to DON and increase the final DON concentration during ensiling.
Have a safe and productive silage harvest season!
Citations
Chibuogwu, M.O., Groves, C.L., Mueller, B., and Smith, D.L. 2024. Effects of fungicide application and corn hybrid class on the presence of Fusarium graminearum and the concentration of deoxynivalenol in ear and stalk parts of corn (Zea mays) used for silage. Plant Disease. https://doi.org/10.1094/PDIS-12-23-2662-RE.
Chibuogwu, M.O., Reed, H., Groves, C.L., Mueller, B., Barrett-Wilt, G., Webster, R.W., Goeser, J., and Smith, D.L. 2025. Influence of hybrid class and ensiling duration on deoxynivalenol accumulation and its derivative deoxynivalenol-3-glucoside while ensiling corn for silage. Plant Disease. https://doi.org/10.1094/PDIS-06-24-1166-RE.