Damon Smith, Extension Field Crops Pathologist, Department of Plant Pathology, University of Wisconsin-Madison
Shawn Conley, Extension Soybean and Small Grains Agronomist, Department of Plant and Agroecosystem Sciences, University of Wisconsin-Madison
It is time for my annual reminder about white mold in soybeans, and its management. The 2023 season in Wisconsin was all about drought and heat. So far, the 2024 season has been on the opposite end of the spectrum. Don’t be complacent about managing white mold this year.

Figure 1. Apothecia, a small mushroom-like structure of the white mold fungus that give rise to spores, which infect soybean flowers.
Remember that the white mold fungus infects soybeans through open and senescing flowers, by spores that are born from small mushroom-like structures called apothecia (Fig. 1). Remember that if the bloom period gets extended due to cool weather, this can lead to an extended window for infection by the fungus. Often cool weather is a double whammy as it is good for the white mold fungus and slows down soybean crop development, thereby extending bloom. This could be at play this season, stay on top of your game!
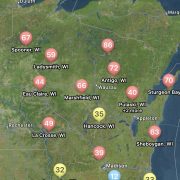
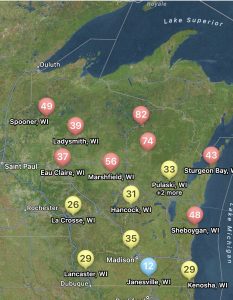
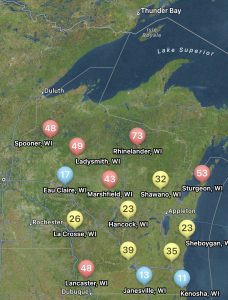
Given the moderate temperatures and moisture we have been getting the risk for white mold is currently moderate to high for the central to northern tiers of the state, while the far southern tier is at generally low risk (Fig. 2).
Predicting White Mold
The flowering growth stages are a critical time to manage white mold in-season. You can view a fact sheet and video on the subject. As you probably know, timing in-season fungicide sprays at the correct time during the soybean bloom period can be extremely difficult. To help solve this decision-making issue, models were developed at the University of Wisconsin-Madison in conjunction with Michigan State University and Iowa State University to identify at-risk regions which have been experiencing weather favorable for the development of white mold apothecia. These models predict when apothecia will be present in the field using combinations of 30-day averages of maximum temperature, relative humidity, and wind speed. Using virtually available weather data, predictions can be made in most soybean growing regions. To facilitate precise predictions and make the model user-friendly, we use the Sporecaster smartphone application for Android and iPhone and also the Field Prophet app for iPhones.
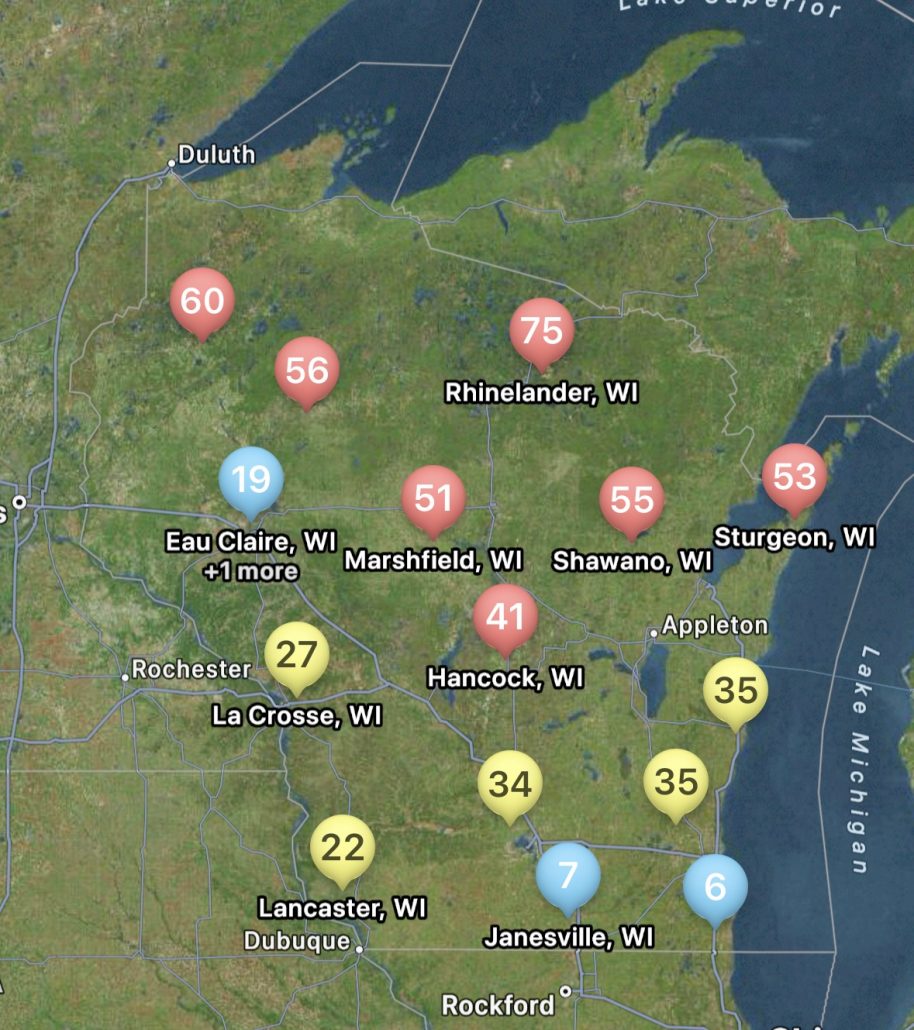
Figure 2. Map of white mold risk generated in the Field Prophet app for Wisconsin, July 8, 2024.
The purpose of the smartphone app is to assist farmers in making early season management decisions for white mold in soybean. The best time to spray fungicides for white mold is during flowering (R1 and R3 growth stages) when apothecia are present on the soil surface. If you have trouble growth-staging soybeans, here is a helpful guide on correctly identifying soybean growth stages.
University research has indicated that the appearance of apothecia can be predicted using weather data and a threshold of percent soybean canopy row closure in a field. Based on these predictions and crop phenology, site-specific risk values are generated for three scenarios (non-irrigated soybeans, soybeans planted on 15″ row-spacing and irrigated, or soybeans planted on 30″ row-spacing and irrigated). Though not specifically tested we would expect row-spacings of 22 inches or less to have a similar probability response to fungicide as the 15-inch row-spacing.
The Sclerotinia apothecial models that underlie the Sporecaster prediction tool have undergone significant validation in both small test plots and in commercial production fields. In 2017, efficacy trials were conducted at agricultural research stations in Iowa, Michigan, and Wisconsin to identify fungicide application programs and thresholds for model implementation. Additionally, apothecial scouting and disease monitoring were conducted in a total of 60 commercial farmer fields in Michigan, Nebraska, and Wisconsin between 2016 and 2017 to evaluate model accuracy across the growing region. Across all irrigated and non-irrigated locations predictions during the soybean flowering period (R1 to early R4 growth stages) were found to explain end-of-season disease observations with an accuracy of 81.8% using the established probability thresholds now programmed in the app. We have made additional improvements for 2024, to further refine accuracy. So if you have used Sporecaster before, you might want to check the version in the “Help and Info” button to be sure you have version 1.41.3 of Sporecaster.
We also know that for highly susceptible soybean varieties, the action threshold should be adjusted down. Research in the upper Mid-west demonstrated that for most soybean varieties the default action threshold depicted in the tool when you set up a field is accurate. However, some varieties are highly susceptible and the action threshold should be moved down from 40% to 20% for varieties that are known to be highly susceptible. this can improve accuracy of the tool and recommendation for fungicide application.
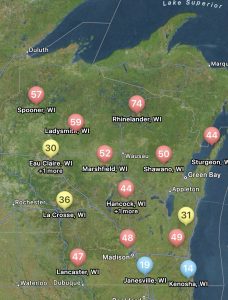
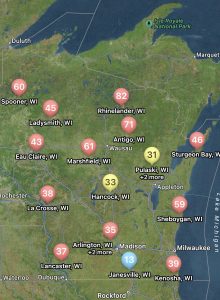
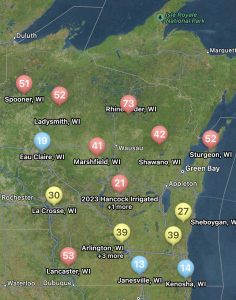
Not only can users run predictions of risk during the soybean bloom period for any field, but you can also set up visual maps to look at multiple sites simultaneously. An example for the state of Wisconsin can be found in figure 2, which represents risk for July 8, 2024, for non-irrigated soybeans. Currently, if soybeans are flowering, risk is moderate-to-high in much of Wisconsin for non-irrigated soybeans. I personally like the Field Prophet version of the prediction tool a bit better as it provides a 7-day historical trend of risk and also provides a 7-day true forecast. The historical trend lines can be viewed on the fly in your field list (Fig. 3). If the trend is moderate, but the slope of the line is increasing, then you might want to keep a close eye on the white mold risk. We find that the trends are much more informative for decision-making vs. just the daily instantaneous risk level.
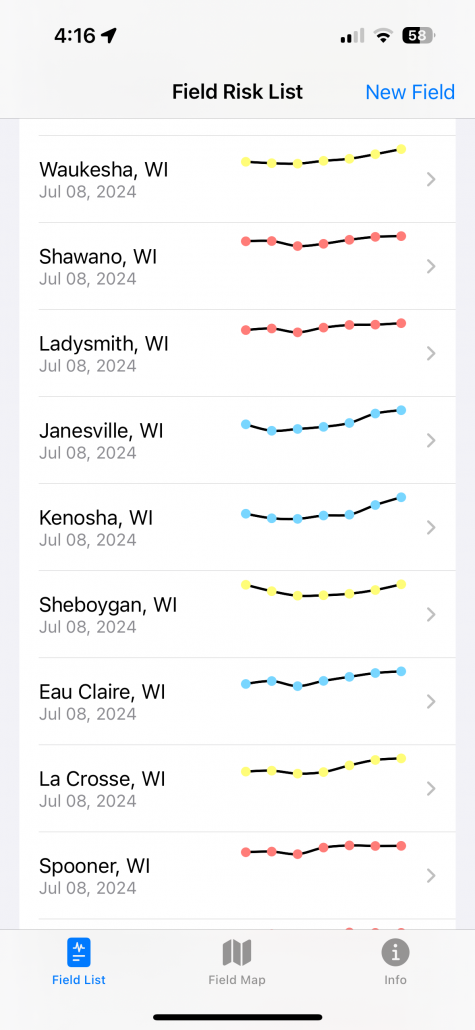
Figure 3. 7-day historical white mold risk trend lines as depicted in the Field Prophet app on July 8, 2024.
What to Spray for White Mold?
If you have decided to spray soybeans for white mold, what are the best products to use? Over the last several years we have run numerous fungicide efficacy trials in Wisconsin and in conjunction with researchers in other states. In Wisconsin, we have observed that Endura applied as a single application at 8 oz at the R3 growth stage performs well. The window to spray runs from R1 to R3, but our recent data suggests waiting a bit into that window improves efficacy of the fungicide application vs. spraying at R1. If you do choose to spray at the R3 growth stage, be sure to focus on getting good canopy penetration with your fungicide spray. Soybean canopies at R3 can be dense and hauling more water, slowing your sprayer speed, and increasing spray pressure can all help improve spray penetration in those dense canopies. Other fungicide options also include Omega and Proline. You can view results of past fungicide evaluations for Wisconsin by CLICKING HERE. If you would like to run tailored estimations of return on investment for various fungicide programs, you can use another smartphone application called Sporebuster.
What is Sporebuster?
When a fungicide application is needed to control white mold in soybeans, Sporebuster can help determine a profitable program. You enter your expected soybean price, expected yield, and treatment cost. Sporebuster instantly compares ten different treatment plans at once to determine average net gain and breakeven probability of each. You can mark, save and share by email, the best plans for your farming operation.
The purpose of Sporebuster is to assist soybean farmers in making a fungicide program decision that is profitable for their operation. Sporebuster is meant to complement Sporecaster. Once Sporecaster recommends a fungicide application, Sporebuster can be used to determine a profitable program.
Information that drives Sporebuster is based on research from 2009-2016 from across the upper Midwestern US. Statistical models were developed based on these data that included white mold pressure and yield response from fungicide for 10 common fungicide programs. Due to recent changes in the markets, be sure to manually adjust the cost of the product you are interested in, so that the tool returns an accurate estimate of return on investment. My advice is to call local suppliers and see what products you can get and what the per acre cost will be to get the application done. Details about the research behind Sporebuster can be found by CLICKING HERE.
Helpful Smartphone Application Links
Sporecaster
- Click here to download the Android version of Sporecaster.
- Click here to download the iPhone version of Sporecaster.
- Click here to download the Field Prophet version of Sporecaster for the iPhone.
Sporebuster
- Click here to download the Android version of Sporebuster.
- Click here to download the iPhone version of Sporebuster.
- Here is a video on how to use Sporebuster and interpret the output.
Other Resources
- To watch an in-depth video on white mold management, CLICK HERE.
- To find more information on white mold, view a web book from the Crop Protection Network, CLICK HERE.
Scientific References
- Willbur, J.F., Fall, M.L., Blackwell, T., Bloomingdale, C.A., Byrne, A.M., Chapman, S.A., Holtz, D., Isard, S.A., Magarey, R.D., McCaghey, M., Mueller, B.D., Russo, J.M., Schlegel, J., Young, M., Chilvers, M.I., Mueller, D.S., and Smith, D.L. Weather-based models for assessing the risk of Sclerotinia sclerotiorum apothecial presence in soybean (Glycine max) fields. Plant Disease. https://doi.org/10.1094/PDIS-04-17-0504-RE
- Willbur, J.F., Fall, M.L., Byrne, A.M., Chapman, S.A., McCaghey, M.M., Mueller, B.D., Schmidt, R., Chilvers, M.I., Mueller, D.S., Kabbage, M., Giesler, L.J., Conley, S.P., and Smith, D.L. Validating Sclerotinia sclerotiorum apothecial models to predict Sclerotinia stem rot in soybean (Glycine max) fields. Plant Disease. https://doi.org/10.1094/PDIS-02-18-0245-RE.
- Fall, M., Willbur, J., Smith, D.L., Byrne, A., and Chilvers, M. 2018. Spatiotemporal distribution pattern of Sclerotinia sclerotiorum apothecia is modulated by canopy closure and soil temperature in an irrigated soybean field. Phytopathology. https://doi.org/10.1094/PDIS-11-17-1821-RE.
- Willbur, J.F., Mitchell, P.D., Fall, M.L., Byrne, A.M., Chapman, S.A., Floyd, C.M., Bradley, C.A., Ames, K.A., Chilvers, M.I., Kleczewski, N.M., Malvick, D.K., Mueller, B.D., Mueller, D.S., Kabbage, M., Conley, S.P., and Smith, D.L. 2019. Meta-analytic and economic approaches for evaluation of pesticide impact on Sclerotinia stem rot control and soybean yield in the North Central U.S. Phytopathology. https://doi.org/10.1094/PHYTO-08-18-0289-R.
- Webster, R.W., Mueller, B., Conley, S.P., and Smith, D.L. 2023. Integration of soybean (Glycine max) resistance levels to Sclerotinia stem rot into predictive Sclerotinia sclerotiorum apothecial models. Plant Disease. https://doi.org/10.1094/PDIS-12-22-2875-RE.